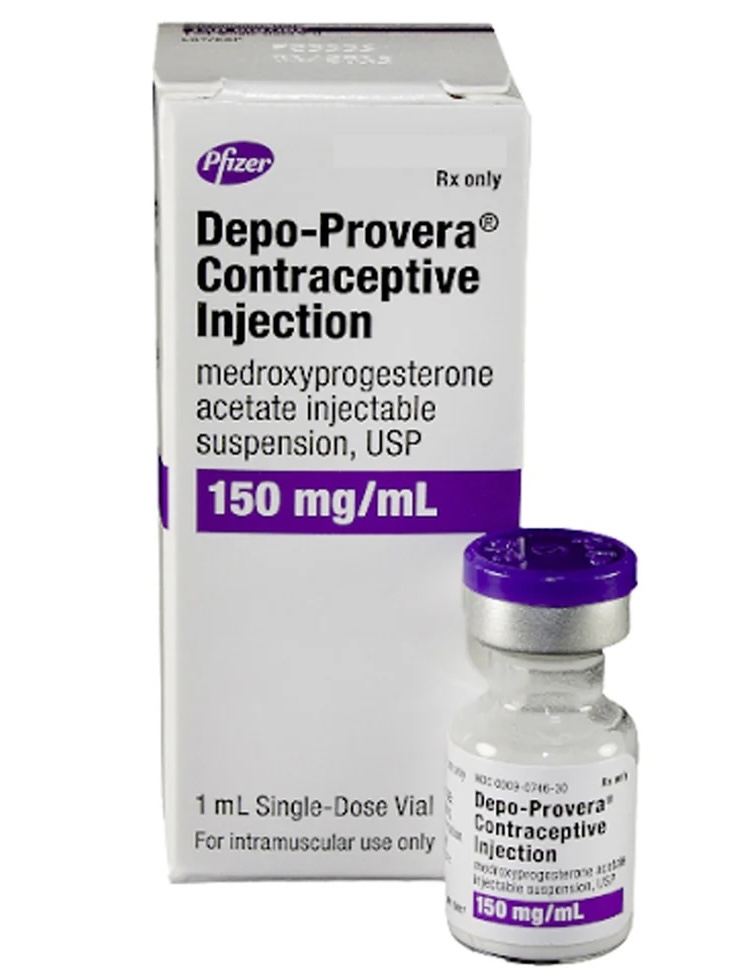
Our firm is in the process of pursuing claims against Pfizer and other defendants related to an apparent increased risk of meningioma, a type of brain tumor, in females that have taken Depo-Provera.
THE CLAIM
Recent studies have shown an increase statistical risk in women that received Depo-Provera injections, specifically 150mg dosage injections. A serious increased risk has not been found generally when the active ingredient, medroxyprogesterone acetate, was given orally. Additional studies are described below.
CRITERA
We are taking cases in certain states where an individual has
- received at least two doses of Depo-Provera, and
- been diagnosed with meningioma brain tumor
TIMELINES
There are time limits such as statutes of limitation and statutes of repose that can limit prevent you from filing a lawsuit so you should contact an attorney promptly if you have a potential claim. Litigation is at an early phase and no trials have yet been completed. Federal court cases may soon be consolidated in what is known as an MDL (multidistrict litigation).
PROCESS
The inital process will be a telephone interview to obtain important information about your claim. We will obtain a HIPAA release from you to verify the type and duration of Depo Provera usage (from physicians and pharmacies) and additionally request information related to meningioma diagnosis.